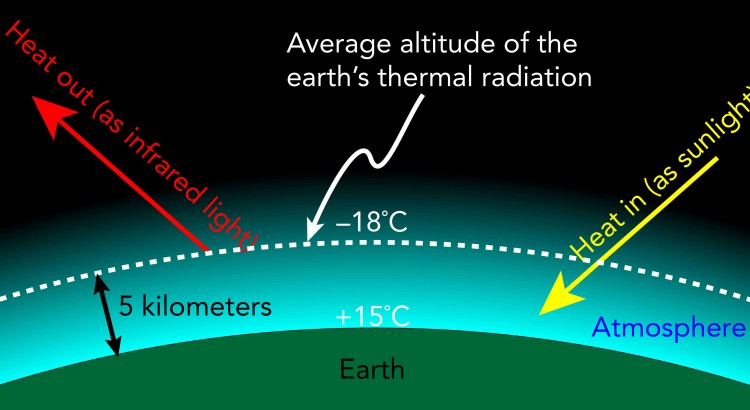
The earth receives heat from the sun at an incredible rate and, if it didn’t get rid of that heat, it would get hotter and hotter. To maintain a steady average temperature, the earth must radiate away heat just as fast as it receives that heat from the sun. In other words, the thermal radiation that the sun emits into empty space must be equal to the thermal radiation the earth receives from the sun.
Though they’re equal in amount, these two thermal radiations have quite different spectrums. Because the sun is extremely hot, its thermal radiation spectrum is largely visible light. The earth, on the other hand, is relatively cool and its thermal radiation spectrum is almost entirely invisible infrared light. While the sun’s thermal radiation is brilliantly visible, we can’t see the earth’s thermal glow or where it’s coming from, which is important because some of it comes from our atmosphere.
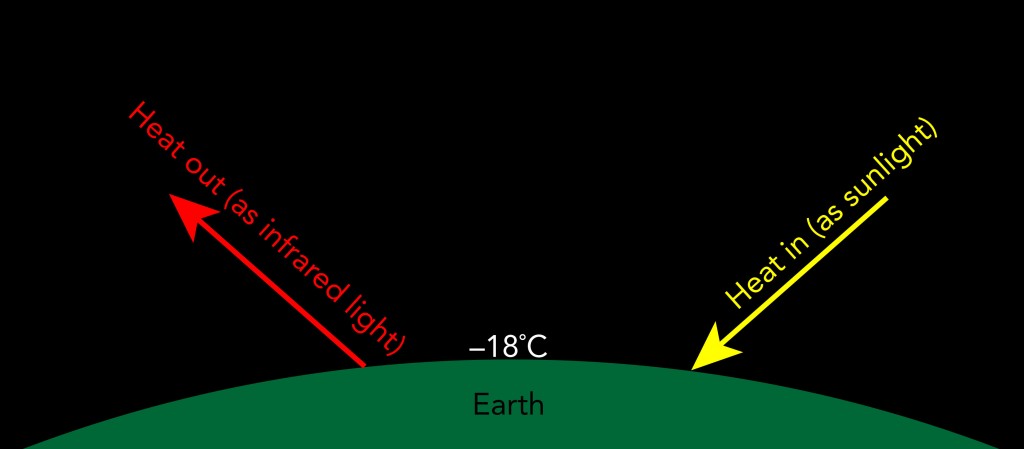
If the earth had no atmosphere, its average surface temperature would be approximately -18 °C. At that temperature, the earth’s surface would emit just enough thermal radiation to balance the thermal radiation it receives from the sun. If the earth were hotter, it would radiate away more heat than it receives and cool down. If the earth were cooler, it would radiate away less heat than it receives and warm up.
But the earth does have an atmosphere and that atmosphere contributes to these exchanges of thermal radiation. Although it’s relatively transparent to visible light, the atmosphere is able to emit and absorb significant portions of the infrared spectrum. As a result, a substantial fraction of the earth’s thermal radiation originates in its atmosphere.
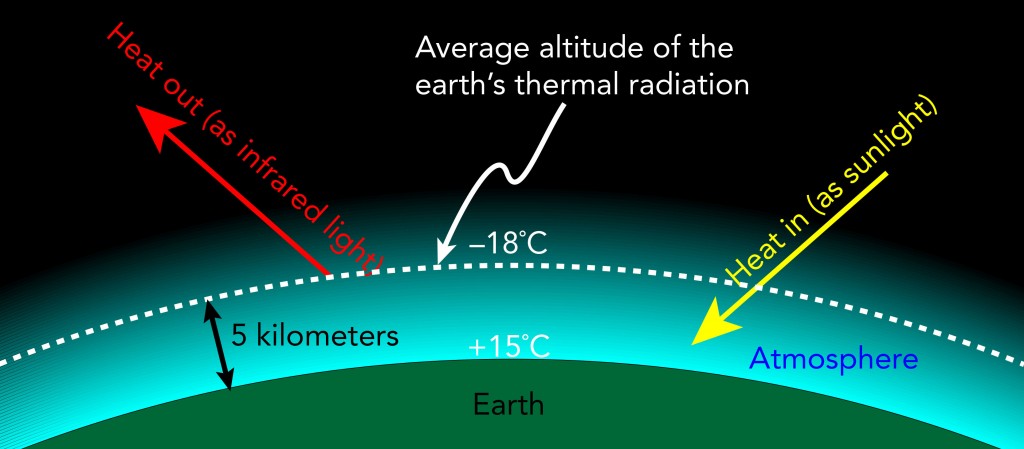
Because of the atmosphere’s contribution to the earth’s thermal radiation, the average altitude at which the earth’s thermal radiation originates is not ground level. Instead, it’s 5 kilometers above sea level, the altitude at which the air temperature is about -18 °C. So the earth’s atmosphere shifts the -18 °C average radiating surface from sea level to an altitude of 5 kilometers above sea level.
If you’ve ever traveled up into the mountains and felt the air during your trip, you’ve probably noticed that air’s temperature decreases with altitude. The earth’s atmosphere has a natural temperature gradient of about -6.6 °C per kilometer upward. A simple explanation for that temperature gradient is that at higher altitudes, air must commit more of its overall energy to gravitational potential energy (energy stored in the force of gravity), leaving it with less for thermal energy (energy associated with temperature).
Since the air temperature increases by 6.6 °C each time you descend 1 kilometer, the air temperature at an altitude of 4 kilometers is -11.4 °C (-18 °C + 6.6 °C), at an altitude of 3 kilometers is -4.8 °C (-11.4 + 6.6 °C), …, and at sea level is 15 °C. Sure enough, the average historical air temperature at sea level is about 15 °C.
Which brings us to the question itself: “Why do greenhouse gases warm the earth?”
The 5 kilometer average altitude of origin for the earth’s thermal radiation actually depends on the atmosphere’s chemical composition. Some molecules in the air, notably nitrogen and oxygen, are remarkably transparent in the infrared and barely contribute to the earth’s thermal radiation. Other molecules, notably water, carbon dioxide, methane, and nitrogen oxides, interact strongly with infrared light and contribute significantly to the earth’s thermal radiation. Those thermally radiating gases are collectively known as “greenhouse gases.” The higher the concentration of those greenhouse gases in the atmosphere, the more the atmosphere contributes to the earth’s thermal radiation and the higher the average altitude of origin for the earth’s thermal radiation.
As human-produced greenhouse gases accumulate in the earth’s atmosphere, the average altitude of origin for the earth’s thermal radiation increases. If that average altitude were to rise from 5 kilometers to 6 kilometers, the average temperature at sea level would increase by another 6.6 °C to 21.6 °C. A rise of that magnitude would be catastrophic or even apocalyptic.
Alas, the average altitude of origin for the earth’s thermal radiation has already risen significantly since the industrial revolution and with it, a rise in the average temperature at sea level. The rate of temperature rise is alarming and the task of halting it or at least slowing it substantially cannot be put off for another generation. Even with serious international effort, it’s likely that many areas of the world will become uninhabitable by the next century, either because they are too hot for human survival or because they are under water as the result of rising sea levels.